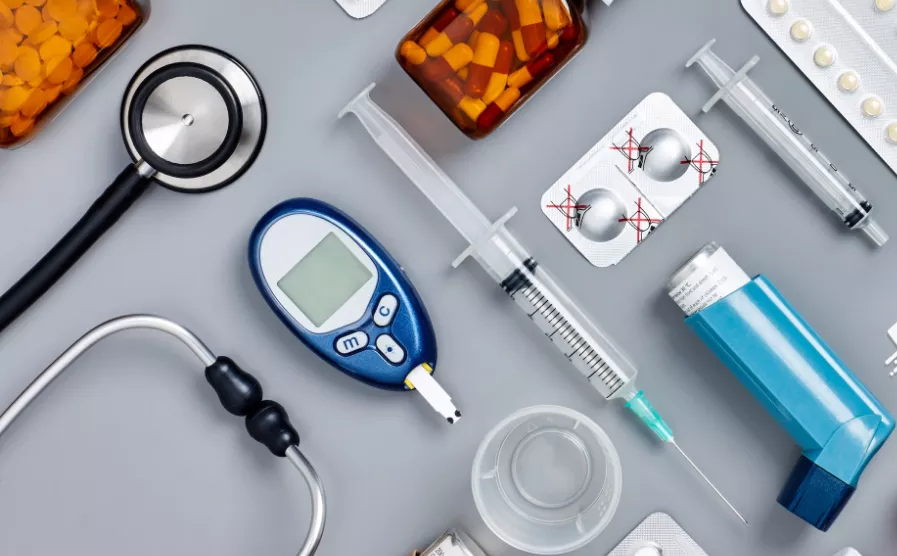
It is very important to note that medical products require strict adherence to regulatory standards and quality control measures, so it is crucial to work with a professional and rich experienced injection molding company to ensure that your medical projects successful. RJC serving many medical companies around the world and some of them are famous of the world. Handle 1000+ molding projects.
Research and gather information about the specific medical product that you want to manufacture using injection molding. This includes understanding the material requirements, design specifications, and regulatory requirements for the products. Checking the data sheet of materials, selecting the appropriate materials and meet ROHS, CE, FDA etc certifications.
Identify a reputable injection molding company that has rich experience in the medical industry to do engineering analysis, and can provide the necessary expertise and capabilities to produce your product. Develop a detailed manufacturing plan that outlines the processes and procedures needed to produce the medical product. This may include designing and fabricating the injection mold, and establishing quality control measures.
Ensure the necessary equipment and facilities to meet the specific requirements of the medical product, including clean rooms and other controlled environments as needed. Work closely supplier to monitor the production process and make any necessary adjustments to ensure that the final product meets the required specifications and regulatory standards.
Test and validate the finished products to ensure the products meet the required performance and safety standards before releasing them for market.
Engineering analysis:
For medical products manufacturing, engineering analysis is necessary, and it’s important step in the process of injection mold manufacture, which one will reduce the risks and helps to ensure that the final product will meet the desired specifications and performance requirements. It allows designers and manufacturers to identify potential issues or challenges in the design and manufacturing process, and to make any necessary changes or modifications to the product design before mold manufacture.
There are several types of engineering analysis that may be performed during the injection mold design and manufacturing process, including:
DFM report: Design for Manufacturing (DFM) report, which is a bridge between product developers and mold makers. It has been used in many manufacturing industries and has proven to be an effective way to increase efficiency. Most medical projects are with top requirements and high cost, So, a comprehensive DFM reporting mold manufacturing project will be the first step to success. As a mold maker, the more potential problems you foresee, the less risk you have in the manufacturing process. This also is a way to avoid money waste.
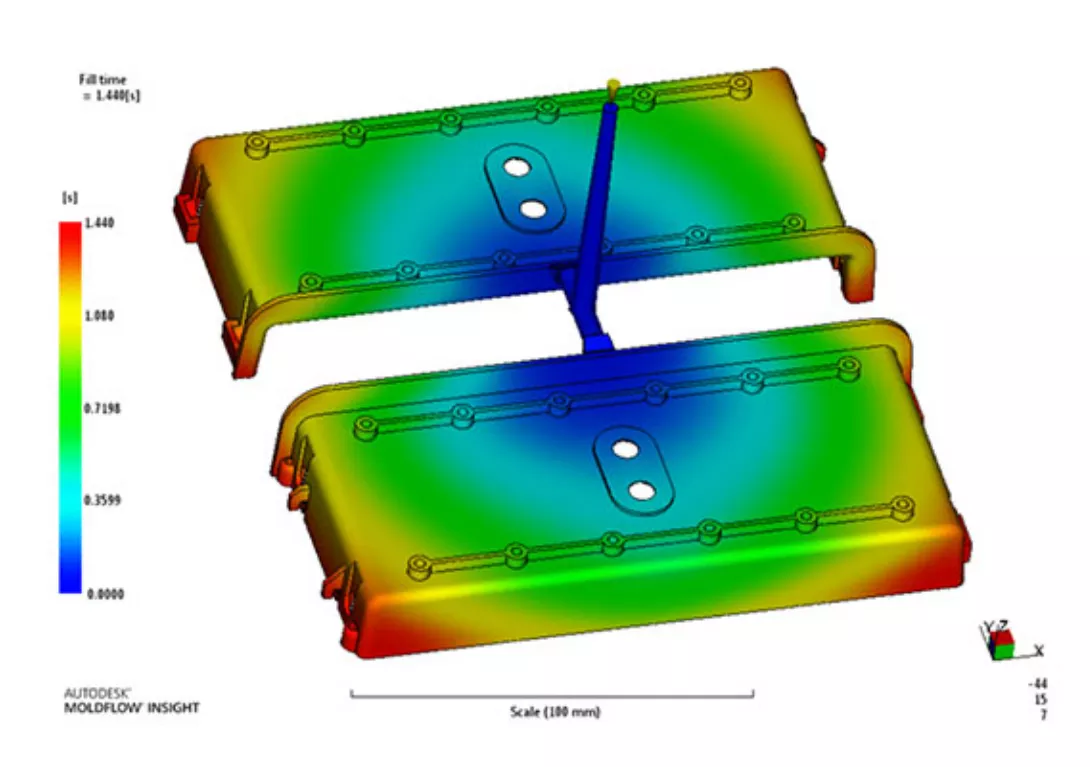
Mold flow analysis: This is a computer simulation that helps to predict how the molten plastic will flow through the mold during the injection molding process. It can be used to identify potential issues with the design of the mold, such as sink area, deformation, air traps, shrinkage etc
Clean room required:
Clean room is controlled environments that are designed to minimize the level of contaminants, such as dust and microorganisms, present in the air. These environments are often used in the production of medical products, including drugs, medical devices, and surgical instruments, because contaminants can have a significant impact on the quality and safety of these products. The use of a dust-free workshop in the production of medical products can help to ensure that these products are of high quality, safe for use, and produced efficiently.
Production managements:
Quality control: It is important to have a robust quality control system in place to ensure that the medical products being produced meet the required standards of quality and safety. And control each production process.
Standard operating procedures (SOPs): Establishing clear SOPs for the production of medical products can help to ensure that the process is consistent and follows best practices.
Training: Proper training of production staff is essential to ensure that they understand the requirements for producing medical products and can follow the established SOPs.
Documentation: Maintaining accurate and thorough documentation of the production process can help to identify any potential issues and facilitate continuous improvement.
Regulatory compliance: It is important to ensure that the production of medical products meets all relevant regulatory requirements. This may include obtaining necessary licenses and permits, as well as following good manufacturing practices (GMPs).
Quality management system (QMS): Implementing a QMS can help to ensure that the production of medical products is consistently of high quality and meets all regulatory requirements. A QMS include elements such as quality control measures, training programs, and documentation processes.ISO9001, ISO13485 are QMS.
Overall, every process is important in order to run medical projects smoothly. RJC has extensive experience in manufacturing medical products in the field of injection molding. Feel free to contact us at any time.